-
In the past a few decades, lasers have been playing an indispensable role in many aspects of our lives, such as CD players, super market barcode readers and pocket laser pointer in our daily life; laser surgery in medicine; metal cutting and defect detection in industry; and laser weapons and ranging-finding, guidance and detection instruments in the military. The widespread use of lasers has increased the risk of accidental or intentional damage to human eyes and optical systems. There have been many reports in the news on the incidence of laser damage to optical systems and military personnel’s eyes; and the potential to use commercial laser system as weapons becomes highly possible[1-4] . Such increased threat has triggered the development of optical limiting materials and devices for eye and sensor protections[5-16].
Generally, an ideal optical limiter should transmit 100% light at low incident laser intensity, but absorb, reflect, scatter or diffract most of the light when the laser intensity reaches the point that may cause damage to human eyes or optical sensors. The response time for the limiter should be faster than 1 ns. A broad spectral response that covers the visible to near-infrared (NIR) spectral range (400-900 nm) for protection of human eyes and night vision devices from the “frequency-agile” laser systems, and a broad temporal bandwidth effective for a variety of pulsed (< 1 ms) or CW (>1 ms) lasers are required. In addition, the material and device should have low toxicity and exhibit long-term stability. It should be able to operate at a variety of environments.
-
Currently, the commercially available devices for eye and sensor protection include fixed-line filters that selectively eliminate 2-3 wavelengths (such as the laser protection goggles), neutral density filters, and mechanical or electro-optical shutters. However, the fixed-line filters have very low linear transmission (usually 10%-20%) and cause color distortion; neutral density filters are lack of contrast due to the overall low linear transmission; while the mechanical/electro-optical shutters have very slow response time and the optical systems are typically complicated. Most importantly, none of these devices can meet the requirement for broadband spectral and temporal responses. Therefore, new materials and device concepts are necessary for the development of new optical devices that can remove the threat of a laser beam but has a minimum impact on the optical system or on one’s capability to commit mission. Meanwhile, the device must have broad spectral and temporal operating bandwidth.
To meet the aforementioned requirements for optical limiters, a variety of nonlinear optical mechanisms have been investigated. Reverse saturable absorption (RSA), two-photon absorption (TPA), free-carrier absorption (FCA), nonlinear refraction (NLR), and nonlinear scattering have been found to be useful for passive reduction of optical transmission (the best approach to counter the frequency agile, short pulse threat)[5-16]. Because the performance of an optical limiter is predominantlydetermined by the properties of the material used in the device, seeking for optical limiting materials that exhibit one or multiple of the aforementioned nonlinear optical properties has been the major theme for the development of optical limiters. To date, the most widely investigated materials include semiconductors, carbon black suspensions, carbon nanotubes and graphene, organic compounds, organometallic complexes, liquid crystals, and organic/inorganic hybrids. Semiconductors typically exhibit low limiting threshold and good optical limiting performance; however, their damage thresholds are usually low. Carbon black suspensions (CBS) show broadband optical limiting extending to the NIR regions, but they must be used in liquid state and are subject to laser degradation. CBSs are not effective for ultrafast laser pulses (ps and fs lasers) either. Organic compounds or organometallic complexes typically possess fast time response and have high damage threshold, and the nonlinear optical properties can be readily tuned via structural modifications. Therefore, they would be better candidates to be developed into broadband optical limiting materials.
-
RSA and TPA are two of the nonlinear optical phenomena in which the absorptivity of the absorber increases with the increased incident fluence or intensity. RSA occurs when a material has stronger excited-state absorption than that of the ground state at the interested wavelengths, which is a fluence-dependent process. TPA takes place when a material absorbs two photons of the same or different energies simultaneously to be populated to a virtue or real excited state of the material, thus the absorptivity depends on the square of the light intensity. TPA is an ultrafast and intensity-dependent process. Materials exhibiting strong RSA and/or TPA have potential applications in optical switching[17], optical limiting[18], laser mode locking[19], optical pulse shaping[20], spatial light modulation[20-21], laser beam compression[22], and TPA-induced photodynamic therapy[23], etc. Optical limiting devices based on RSA and/or TPA have the advantages of simple device design, broadband spectral and temporal responses.
For an ideal reverse saturable absorber, the molecule should have low but measurable ground-state absorption in the interested wavelength to populate the excited state; while the excited-state absorption cross section should be much larger than that of the ground state. The lifetime of the excited-state should be longer than the laser pulsewidth. For RSA of ns or longer laser pulses, a high triplet quantum yield is desired because the triplet excited-state absorption is the major contributor for absorbing longer pulsewidth laser beams. To meet these criteria, the conjugation length of the molecules should be carefully tuned because large π-conjugation would red-shift and increase the ground-state absorption cross sections in the visible spectral regions and thus reduce the ratios of the excited-state absorption cross section with respect to that of the ground state, which is a critical parameter for RSA. On the contrary, in order to increase the TPA crossssections of organic molecules, the molecules should possess extensive π-framework and/or strong intramolecularcharge transfer characters, which could decrease the transparency of the materials in the visible spectral regions. In addition, although the two-photon absorbing dyes can be almost 100% transparent in the NIR regions at low intensity, they usually only work for short-pulsewidth laser sources (ps or fs), not for longer pulsewidth lasers. Therefore, developing organic moleecules that have light color (weak or no ground-state absorption in the spectral regions of 450-900 nm), but exhibit broad and strong excited-state absorption in the 450-900 nm regions and reasonable TPA in the NIR regions is desired. In these molecules, RSA could occur in the green to red spectral regions; while two-photon induced excited-state absorption could induce optical limiting in the far-red to NIR regions. Combination of these nonlinear absorption phenomena could generate broadband optical limiting materials.
In organometallic complexes, the interactions between the metal center and the organic ligand generate multiple charge transfer excited states, which give rise to broad excited-state absorption. Selection of appropriate organic ligand could keep the major ground-state absorption bands to <450 nm. Meanwhile, heavy transition-metal complexes could exhibit high yield of triplet excited-state formation due to the heavy-atom enhanced intersystem crossing (ISC). Therefore, they are promising candidates for broadband optical limiting.
Among the variety of organometallic complexes that exhibits RSA, metallophthalocyanines possessing strong RSA in the visible spectral regions, such as leadphthalocyanines (PbPc) or silicon naphthalocyanine (SiNc), are among the most promising ones. However, the strong linear absorption in the red to NIR regions prevents their application as broadband optical limiting materials in the NIR regions. To solve this problem, square-planar platinum(Ⅱ) complexes[24-25] may be good candidates.
-
Square-planar d8 Pt(Ⅱ) complexes are interesting heavy transition-metal complexes with potential applications in DNA intercalation[26], protein probing[27], chemosensing[28], photovoltaic cells[29], light-emitting devices[30], catalysis[31], and optical limiting[32]. Figure 1 shows some representative structures for the most common types of square-planar Pt(Ⅱ) complexes, including Pt(Ⅱ)-bisphosphine bisacetylide complexes, Pt(Ⅱ) bipyridine bisacetylide complexes, and Pt(II) terdentate acetylide complexes. Among these complexes, Pt:ethynyl complexes are among the most well studied Pt(Ⅱ) complexes for nonlinear optics and optical limiting[32-38]. Unfortunately, these complexes suffered from instability upon laser irradiation, which limits their applications in practical optical limiting.
In contrast, Pt(Ⅱ) terdentate or diimine complexexhibit excellent thermal and photochemical stabilities due to chelation of Pt(Ⅱ) ion by the terdentate or bidentate ligands. In addition, like the Pt:ethynyl complexes, the Pt(Ⅱ) terdentate or diimine complexes possess low ground-state absorption but strong excited-state absorption (ESA) in most of the visible to the NIR regions. The heavy-atom effect of the Pt(Ⅱ) ion facilitates the intersystem crossing and gives rise to a high triplet excited state population upon excitation, which would enhance the triplet excited-state absorption. Moreover, it is facile to conduct structural modifications on these complexes. The type of terdentate ligand, the 4′-substituent on the terdentate ligand, and the monodentate co-ligand can be readily altered. The terdentate ligand could be assembled with other organic[39-41] or inorganic chromophores[42] and be used as building blocks for dendrimers[43-44]. It is also possible to assemble the mononuclear motif into di-, tri- and multinuclear complexes through a variety of bridging ligands[45-49]. Therefore, since our first report on the RSA-based optical limiting of Pt(Ⅱ) terdentate oligophenylacetylide complexes[50], our group and other groups have conducted an extensive study on the RSA/optical limiting and/or TPA of Pt(Ⅱ) terdentate complexes[51-77]. While the most common terdentate ligands include 2,2′:6',2″-terpyridine (N^N^N), 6-phenyl-2,2′-bipyridine (C^N^N), or 2,6-biphenylpyridine (C^N^C), this minireview will only focus on the RSA/optical limiting and/or TPA of Pt(Ⅱ) terpyridine complexes.
-
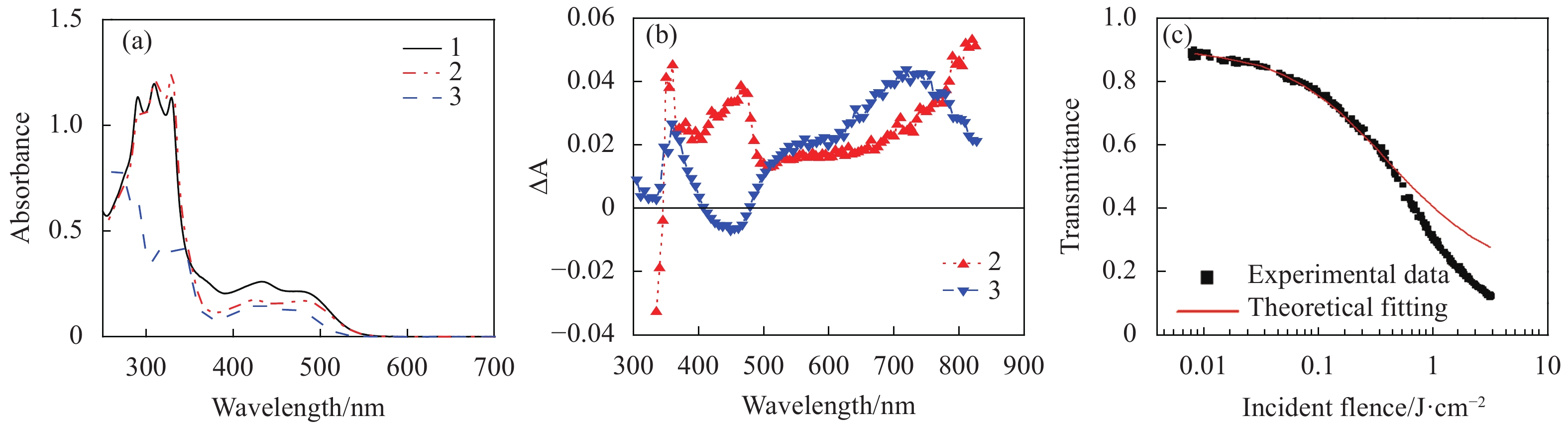
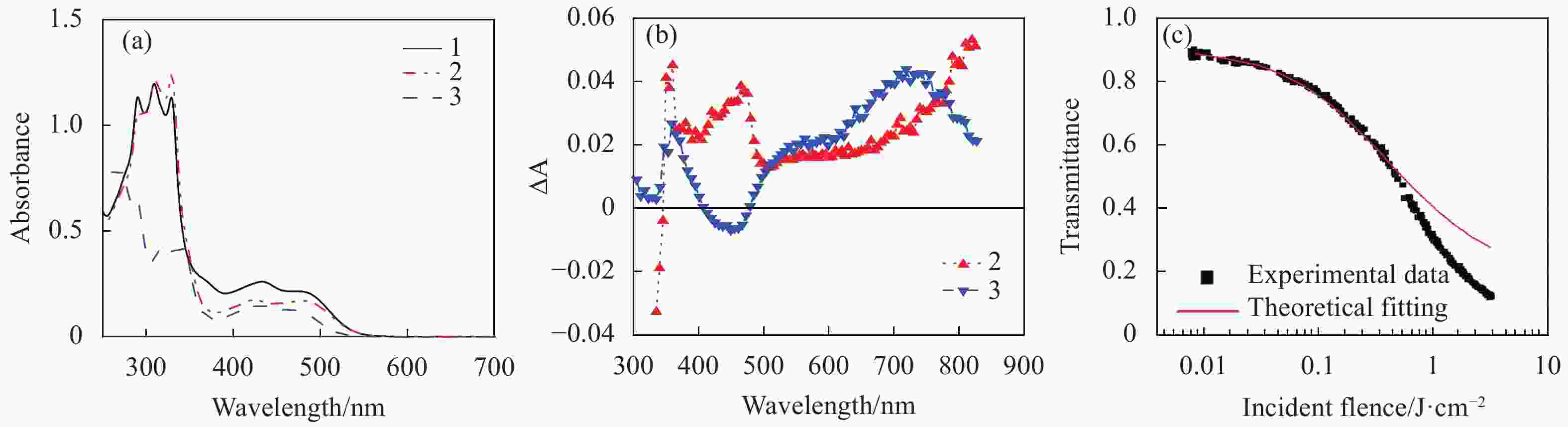
The first study on the RSA-based optical limiting of Pt(Ⅱ) terpyridine acetylide complexes (1-3 in Fig.2) was reported by our group[50]. Those complexes contain oligophenylacetylide ligands with different degrees of conjugation and different substituents on the terpyridine ligand. The linear absorption spectra of the complexes (Fig.3(a)) exhibited strong absorption bands in the UV region (260-380 nm) that were assigned to 1π,π* transitions within the ligands; and broad bands in the 380-550 nm regions that were attributed to metal-to-ligand charge transfer (1MLCT) transitions. Above 550 nm, the complexes were essentially transparent. Therefore, a broad optical window was formed at >500 nm, whereas the triplet excited state exhibited a broad absorption band (Fig.3(b)). Optical limiting of these complexes was demonstrated at 532 nm for 5 ns laser pulses. With a linear transmission of 90% in a 2 mm cuvette at 532 nm, the limiting thresholds (Fth) (defined as the incident fluence at which the transmission started to deviate from the linear transmission) for these complexes were found to be 20-30 mJ/cm2; while the transmission decreased to 12-32% at the incident fluence of 3.6 J/cm2 (Tab.1).
Table 1. Optical limiting parameters at 532 nm for ns laser pulsesa
Complexes Fth/J·cm−2 Fthroughput/J·cm−2 Tlim(at 3.6 J·cm−2) 1 0.03 1.07 0.30 2 0.03 1.16 0.32 3 0.02 0.45 0.12 aModified from Ref. [50] with permission. Copyright © American Institute of Physics 
Figure 2. Structures of Pt(Ⅱ) terpyridine acetylide complexes 1-22 with different substituents on the acetylide ligand or on the terpyridine ligand

Figure 3. (a) Linear absorption spectra of complexes 1-3 (8.8×10−5 mol/L) in CH2Cl2/CH3OH (8∶2) in a 2 mm cell, (b) triplet transient difference absorption (TA) spectra of 2 (2.39×10−5 mol/L) and 3 (2.09×10−5 mol/L) in a 1-cm cell. The TA spectrum of 1 was similar to that of 2, thus was not shown in Fig.3(b), (c) Z-scan experimental data and fitting curve for 3 in CH2Cl2/CH3OH (8∶2) with a concentration of 3×10−4 mol/L and a linear transmission of 95% at 532 nm in a 1 mm cell. Figures a and b are modified from Ref. [50] with permission, copyright © American Institute of Physics
Both the linear absorption spectra and the transient difference absorption spectra are influenced by the degree of π-conjugation in the acetylide ligand. The complexes containing bis(phenylacetylide) ligand (1 and 2) exhibited a broader (380-830 nm) but slightly weaker excited-state absorption in the visible spectral region (500-780 nm) in contrast to 3 that possessed a monophenylacetylide ligand. Due to the weaker ground-state absorption at 532 nm for 3 but stronger excited-state absorption at this wavelength than those for 1 and 2, complex 3 exhibited stronger optical limiting at 532 nm.
To quantitatively understand the excited-state cross section of 3, Z-scan experiment was carried out for 3 at 532 nm using ns laser pulses, and the result was fitted by a five-energy-level model[57], which yielded a value of 5.5×10−17 cm2 for the first triplet excited state absorption cross section (σT1), and is ~ 40 times as large as that of the ground state absorption (σ0=1.4×10−18 cm2). At high fluence (> 0.4 J/cm2), the theoretical curve began to deviate from the experimental data, suggesting the presence of other nonlinear process. However, contribution from the nonlinear scattering was ruled out.
To evaluate the effects of arylacetylide ligand, our group studied a series of 4′-tolylterpyridine Pt(Ⅱ) complexes bearing different arylacetylide ligands (4-10 in Fig.2
)[52]. The photophysical properties and optical limiting performance of these complexes were systematically investigated. These complexes all exhibited intense ligand-localized 1π,π* absorption bands below 370 nm, and broad, moderately strong metal-to-ligand charge transfer (1MLCT)/ligand-to-ligand charge transfer (1LLCT) bands at 370-650 nm (Figs.4(a) and (b)). The separation of 1MLCT band and 1LLCT band became more salient when the electron-donating ability of the substituents at the phenyl ring increased and the 1LLCT band red-shifted (see Fig.4(a) for 7 and 8). However, only complexes 4-6 and 10 showed broad triplet excited-state absorption, with 4 and 5 possessing a narrower band at 350-430 nm and a broad band at 520-820 nm (Fig.4(c)). The transient absorption (TA) of 6 is much weaker, with only a broader band at 500-820 nm. In contrast, the TA of 10 is broader, with positive absorption bands appearing at 380-820 nm. The excited-state absorption of 7, 8, and 9 was too weak to be measured. Due to the lack of ground-state absorption of 6 at 532 nm and the non-detectable excited-state absorption of 7, 8, and 9, optical limiting performance of only complexes 4, 5 and 10 in CH3CN at 532 nm was demonstrated. With a linear transmission of 70% in a 2 mm cuvette at 532 nm, the limiting threshold (defined as the incident fluence at which the transmission dropped to 90% of the initial linear transmission, i.e. at T/T0 = 90%) varied from 0.049 J/cm2 for 4, 0.144 J/cm2 for 5, to 1.09 J/cm2 for 10; whereas the transmission dropped to 28% for 4, 34% for 5, and 44% for 10 (Fig.4(e) and Tab.2). The trend of the optical limiting strength 4>5>10 at 532 nm paralleled the trends of the triplet quantum yields (88±7% for 4, 77±8% for 5, and 60±5% for 10), and of the ratios of the effective excited-state absorption cross section (σeff) vs. ground-state absorption cross section (σg) at 532 nm for these complexes (Tab.2). It appeared that introducing electron-donating substituents (OCH3 and N(CH3)2) on the phenylacetylide ligands diminished the triplet excited-state absorption and the optical limiting of the Pt(Ⅱ) terpyridine arylacetylide complexes. Table 2. Photophysical parameters and optical limiting data for 4, 5, 10, 11, 12, and 19-22 in acetonitrile[51,53]
Complexes τT/ns σ0 c/10−19 cm2 Fth d/mJ·cm-2 Tlime σeff/σ0 4 766a 2.43 48 0.28f, 0.25g >3.57f, >3.89g 5 659a 7.18 144 0.34f >3.02f 10 672a 11.2 1090 0.44f >2.30f 11 62a 2.50[57] 62 0.19f >4.66f 12 51a 2.40 900 0.45f >2.24f 19 255b 18.1 250 0.27g >3.67g 20 408b 4.30 370 0.27g >3.67g 21 384b 17.6 490 0.32g >3.19g 22 2540b 13.4 52 0.18g >4.81g aTriplet excited-state lifetime deduced from the decay of the TA at 700 nm, from Ref.[51]. bTriplet excited-state lifetime deduced from the decay of the TA at 680 nm, from Ref.[53]. cGround-state absorption cross section at 532 nm, from Refs. [51], [53] or [57]. dRSA threshold when the transmission dropped to 90% of the linear transmission. eNonlinear transmittance at high incident fluence. fAt incident fluence of 2.5 J/cm2. gAt incident fluence of 3.0 J/cm2. This table is modified from Refs.[51] and [53] with permission. Copyrights © Chinese Optical Society and American Chemical Society, respectively 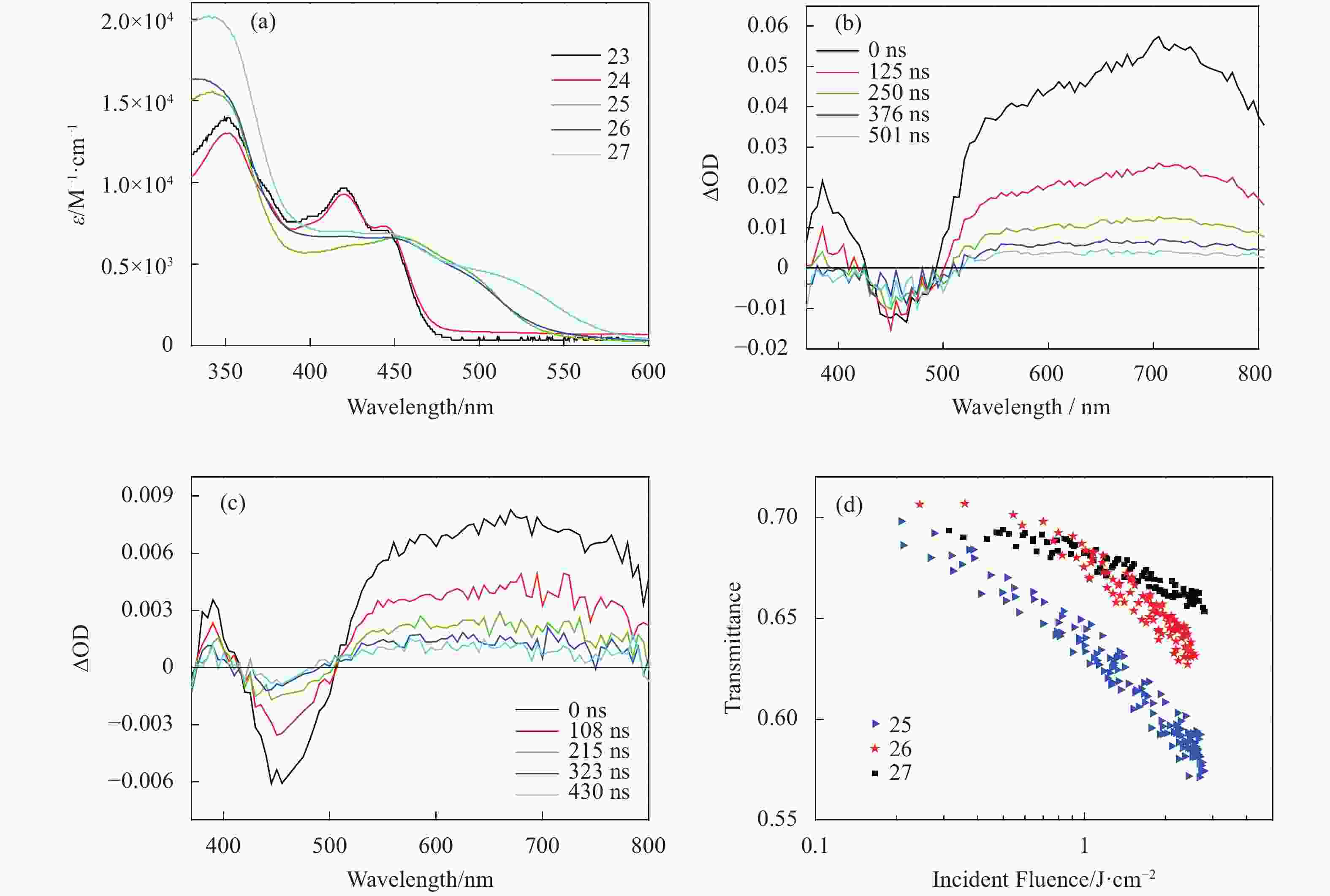
Figure 4. (a) and (b) Linear absorption spectra of complexes 4-12 (1×10−5 mol/L) in acetonitrile in a 1 cm cuvette, (c) ns TA spectra of 4, 5, 6 and 10 in acetonitrile in a 1 cm cell (A = 0.4 at 355 nm). See Ref. [51] for the ns TA spectra of 11 and 12 in acetonitrile, (d) time-resolved fs TA spectra of 11 in acetonitrile, (e) optical limiting curves of 4, 5, 10, 11 and 12 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% in the 2 mm cell. Figures are modified from Refs. [51] and [52] with permission, copyright © Chinese Optical Society and American Chemical Society, respectively
In the work reported by Pritchett and Sun et al, the excited-state absorption of a 4-tolylterpyridyl Pt(Ⅱ) pentynyl complex 11 was investigated using Z-scan technique with ps and ns laser pulses at a variety of pulse energies at 532 nm[57]. This complex exhibited 1π,π* absorption bands below 360 nm, and 1MLCT/1LLCT bands at ca. 430 nm in CH3CN, with a very low ground-stateabsorption cross section of 2.5×10−19 cm2 at 532 nm. On the other hand, both the singlet and triplet excited-state absorption of 11 was broad and strong in the regions of 470-800 nm (see Refs. [51] and [57] and Fig.4(d)). The singlet excited-state lifetime deduced from the decay of fs TA was (268±87) ps, while the triplet excited-state lifetime was 62 ns obtained from fitting the decay of ns TA signals. The triplet excited-state formation quantum yield was determined to be 0.16. By fitting the open-aperture picosecond and nanosecond Z-scan data using a five-level dynamic model, the singlet and triplet excited-stateabsorption cross sections were obtained to be 3.5×10−17 cm2 and 4.5×10−17 cm2, respectively. These values correponded to σS/σ0 of 140 and σT/σ0 of 180. The large ratio of σT/σ0 made complex 11 a very strong optical limiting material for ns laser pulses at 532 nm. For an acetonitrile solution of 11 with a linear transmission of 70% in a 2 mm cuvette at 532 nm, 11 gave rise to the strongest optical limiting among complexes 4, 5, 10, 11 and 12, with a limiting threshold of 62 mJ/cm2 (Fth at T/T0 = 90%) and the transmission dropping to 19% at the incident fluence of 2.5 J/cm2 for 11[57].
Zhu and Liu’s group extended the study of different aryl substituents at the acetylide ligand on the RSA and optical limiting of the Pt(Ⅱ) 4′-phenylterpyridine complexes (13-18 in Fig.2)[74]. Electron-withdrawing or donating aromatic substituents caused a blue- or red-shift of the low-energy 1MLCT/1LLCT absorption bands in these complexes, respectively. However, the electron-donating 4-carbozolylphenyl or 4-diphenylaminophenyl motifs quenched the triplet excited-state absorption in complexes 16-18; whereas the electron-withdrawing nitrophenyl or naphthylimidyl substituent reduced the triplet excited-state absorption coefficients and decreased the triplet excited-state formation quantum yields in 14 and 15. Complexes 13-15 exhibited weak to moderate optical limiting at 532 nm, with a trend of 13>15>14. For complex 13 that gave the strongest optical limiting, the limiting threshold at T/T0 = 70% was found to be 0.30 J/cm2, and the output fluence decreased to 0.97 J/cm2 when the incident fluence reached ~2.5 J/cm2.
To study the effects of substitution at the terpyridine ligands, our group reported a series of Pt(Ⅱ) 4′-aryl-terpyridine phenylacetylide complexes with 4′-naphthyl, 4′-phenanthryl, 4′-anthryl, and 4′-pyrenyl substituents (19-22 in Fig.2
)[53]. Similar to the other Pt(Ⅱ) terpyridine complexes 1-18, these complexes possessed broad and moderately strong 1MLCT/1LLCT transitions at 390-525 nm. Different aryl substituents did not impact the energies of this band significantly (Fig.5(a)). However, both the TA spectral features and the triplet excited-state lifeitmes of these complexes were influenced by the aryl substituents drastically (Fig.5(b)). Complexes 21 and 22 possessed a broader TA at 380-830 nm than those for 19 and 20 (470-830 nm), with the maximum TA band apearing at approximately 520 nm for 22. For 22, its triplet excited state was found to have predominant 4′-pyrenylterpyridine ligand based intrali-gand charge transfer (3ILCT)/3π,π* characters, resulting in a much longer triplet excited-state lifeitme of 2.54 μs. All complexes exhibited optical limiting for 4.1 ns laser pulses at 532 nm in CH3CN solutions with T0 = 70% in a 2-mm cuvette at 532 nm, with 22 giving rise to the strongest optical limiting (Fig.5(c) and Tab.2). The limiting threshold of 22 was 52 mJ/cm2 at T/T0 = 90%, and the transmission decreased to 18% at the incident fluence of 3.0 J/cm2. The strong optical limiting of 22 was ascribed to its much longer triplet excited-state lifetime and the stronger transient absorption at 532 nm. 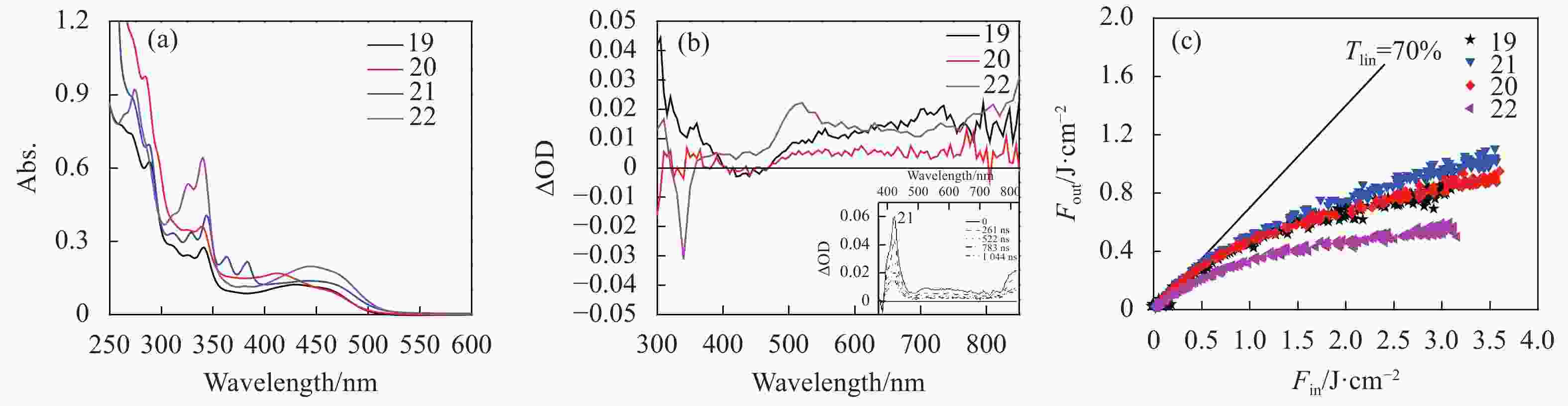
Figure 5. (a) Linear absorption spectra of 19-22 (2.0×10−5 mol/L) in acetonitrile in a 1 cm cuvette, (b) ns TA spectra of 19-22 in acetonitrile in a 1 cm cell (A = 0.4 at 355 nm), (c) optical limiting curves of 19-22 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% at 532 nm in the 2 mm cell. Figures are modified from Ref. [53] with permission, copyright © American Chemical Society
For broadband optical limiting applications, it is important to broaden the ground-and excited-state absorption to longer wavelengths. Because the lowest-energy transition of an Pt(Ⅱ) terpyridine complex typically is the 1MLCT state and the lowest unoccupied molecular orbital (LUMO) is localized at the terpyridine ligand, one of the approaches to red-shift the ground-state absorption is to lower the energy of the LUMO. One possible solution is to increase the coplanarity between the 4-aryl substituent and the central aza-aromatic ring at the terdentate ligand. Our group replaced the central pyridine ring with a 1, 3, 5-triazine ring on the terpyridine ligand for complexes 23-27 (Fig.6), which contained different monodentate ligands and different counter-anions[59]. Because of the electron-deficient nature of the triazine ring and the extension of π-conjugation between the terdentate ligand and the 4-(p-tolyl) substituent on the triazine ring associated with the increased coplanarity, these complexes exhibited bathochromic shifts in their UV-vis absorption spectra compared to their corresponding Pt(Ⅱ) terpyridine complexes (Fig.7(a)). It was found that the increased ligand field strength of the pentynyl or phenylacetylide ligand red-shifted the low-energy transitions in complexes 25-27 compared to those in 23 and 24, which was the results of the red-shifted 1MLCT transition and the occurrence of 1LLCT transition. However, only complexes 25 and 26 exhibited broad and moderate triplet excited-state absorptions from 510 to 820 nm (Figs.7(b) and 7(c)). Nonlinear transmission experiment at 532 nm revealed that only 25-27 exhibited very weak optical limiting at 532 nm, with the optical limiting strength follows the trend of 25>26>27. The stronger optical limiting of 25 was related to its lower ground-state absorption cross section (4.61×10−18 cm2) but a higher triplet excited-state absorption cross section (1.20×10−17 cm2) compared to those of 26 (5.25×10−18 cm2 and 6.48×10−18 cm2 for σ0 and σT, respectively), which resulted in a higher ratio of σT/σ0 for 25 (2.60) vs. that for 26 (1.23). For 27, its much larger ground-state absorption cross section (1.28×10−17 cm2) but non-detectable triplet excited-state absorption led to a much weaker optical limiting at 532 nm. Interestingly, although the counteranion showed a negligible effect on the energies of the 1MLCT/1LLCT states in 25 and 26, it exerted a pronoun-ced effect on the 3MLCT state, reflected by the differences in emission efficiency (0.0007 for 25 vs. 0.0018 for 26), the triplet excited-state extinction coefficient (εT (705 nm) = 3400 M−1·cm−1 for 25 vs. εT (705 nm) = 690 M−1·cm−1 for 26), and the triplet quantum yield (0.21 for 25 and 0.83 for 26), as well as on the optical limiting performance of these two complexes at 532 nm.

Figure 7. (a) Linear absorption spectra of 23-27 in CH2Cl2, (b) ns time-resolved TA spectra of 25 in CH2Cl2 in a 1 cm cell (6.2×10−5 mol/L (A355 = 0.888)), (c) ns time-resolved TA spectra of 26 in CH2Cl2 in a 1 cm cell (3.9×10−5 mol/L (A355 = 0.564)), (d) optical limiting curves of 25-27 in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% at 532 nm in the 2 mm cell. 25 and 26 were dissolved in CH2Cl2, and 27 was dissolved in DMF. Figures are modified from Ref. [5] with permission, copyright © American Chemical Society
Another approach for improving the coplanarity within the N^N^N ligand is to replace the 4′-phenyl substituent on terpyridine ligand by a 4′-pyrimidyl substituent. Our group reported the photophysics and optical limiting of a series of 4′-(5′′′-R-pyrimidyl)-2,2′;6′,2″-terpyridine platinum(Ⅱ) phenylacetylide complexes (28-32 in Fig.6)[56]. These com-plexes possessed moderately intense 1MLCT/1LLCT transitions at ca. 400-500 nm in their UV-vis spectra, which were red-shifted in comparison to those of their corresponding 4′-toly-2,2′:6′,2″-terpyridyl platinum(Ⅱ) phenylacetylide complex, and influenced by the 5′′′ substituents (Fig.8(a)).They also exhibited a linear correlation with the Hammett σp constant of the 5′′′ substituent. These features demonstrated electron delocalization to the pyrimidyl substituent due to improved coplanarity between the pyrimidyl substituent and the terpyridine ligand. All complexes exhibited broad (500-820 nm) and strong singlet and triplet excited-state absorption with the absorption band being maximized at 720-785 nm (Fig.8(b)). Similar to the trend observed from the UV-vis absorption spectra, electron-donating substituent caused blue-shifted excited-state absorption (685 and 720 nm for singlet and triplet excited-state absorption, respectively) but a longer triplet lifetime (660 ns) in 30; while electron-withdrawing substituent induced red-shifts (766 and 785 nm for singlet and triplet excited-state absorption, respectively, Tab.3) of the excited-state absorption and shortened the triplet lifetime (130 ns) in 32. The singlet excited-state lifetimes were reported to be in the range of 37-139 ps for 28-32[63]. Except for 30 (ΦT = 0.19), the other four complexes exhibit high quantum yields of the triplet excited-state formation (0.53-0.66, see Tab.3). All complexes exhibited a moderate optical limiting at 532 nm for 4.1 ns laser pulses. The strength of the optical limiting followed the trend of 29≈28≈31>32≈30.
Table 3. Photophysical parameters of 28-32 in CH3CN
28 29 30 31 32 λabs/nma 463 436 456 463 470 λS1-Sn/nmb 717 685 712 719 766 τs/psc 37±23 56±17 139±128 42±8 46±16 λT1-Tn/nmd 725 720 755 730 785 τT/nse 420 660 130 340 130 ΦTf 0.65 0.53 0.19 0.64 0.66 τisc/psg 57 106 732 66 70 a 1MLCT/1LLCT band maxima. bFemtosecond (fs) TA band maxima. cSinglet excited-state lifetimes. dNanosecond (ns) TA band maxima. eTriplet excited-state lifetimes. fQuantum yields of the triplet excited-state formation. gIntersystem crossing (ISC) time. Data in rows 2 and 5-7 are from Ref. 56; data in rows 3, 4 and 8 are from Ref.[63], copyright © American Chemical Society and Old City Publishing, Inc., respectively 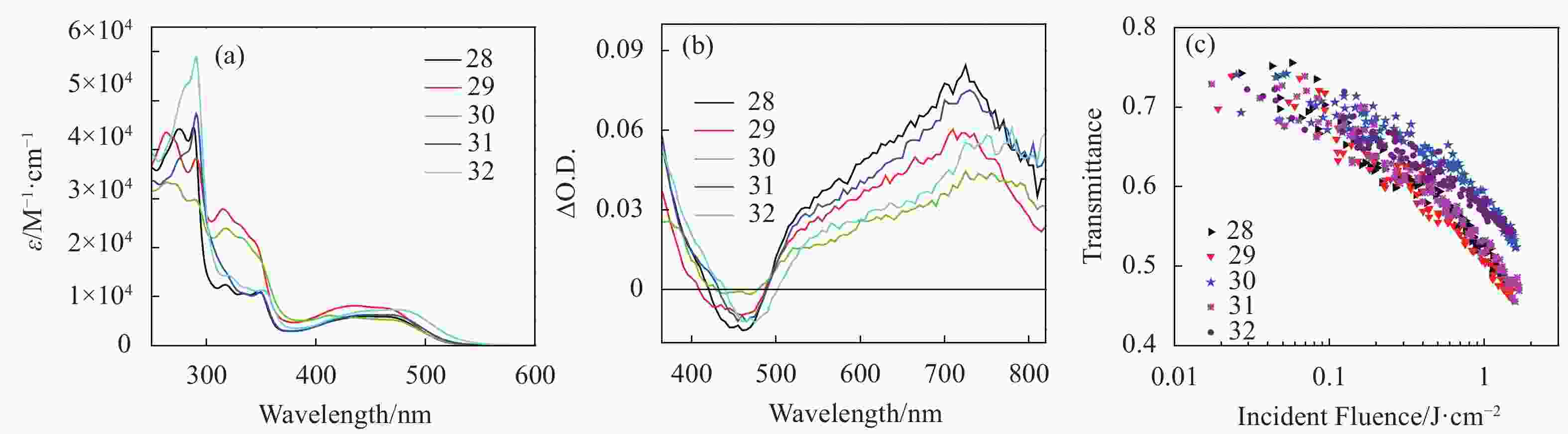
Figure 8. (a) Linear absorption spectra of 28-32 in acetonitrile, (b) ns TA spectra of 28-32 in acetonitrile in a 1 cm cell (λex = 355 nm), (c) optical limiting curves of 28-32 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 75% at 532 nm in the 2 mm cell. Figures are modified from Ref.[56] with permission, copyright © American Chemical Society
Open-aperture Z-scan experiments using ns and ps lasers were carried out for 28-32 in CH3CN solution at 532 nm, and the wavelength dispersion characteristics of 28 were investigated using ps laser pulses over the range of 500-600 nm[63]. Fitting the experimental Z-scan data using a five-level dynamic model gave rise to the singlet and triplet excited-state absorption cross sections (σs and σT, respectively) for 28-32 at 532 nm, which were in the range of (18±1)×10−18−(50±5)×10−18 cm2 for σs and (11±1)×10−18−(14±2)×10−18 cm2 for σT, and corresponded to σs/σ0 ratios of 6.5-29.8 and σT/σ0 ratios of 2.8-11.2 (Tab.4). Complexes 28, 29 and 31 exhibited larger ratios of σs/σ0 and σTΦT/σ0 than those of 30 and 32. These trends corresponded to the optical limiting trend for these complexes at 532 nm. In addition, the σs/σ0 ratios of 28 increased drastically from 1.9 at 500 nm to 260 at 600 nm (Tab.5), implying that this complex could exhibit much stronger optical limiting at longer visible wavelengths.
Table 4. Ground-state and excited-state absorption cross sections of 28-32 in CH3CN at 532 nm
σ0a/10−18 cm2 σTb/10−18 cm2 σT/σ0 σTΦT/σ0 σsc/10−18 cm2 σs/σ0 σTd/10−17 cm2 σT/σ0 28 1.30 60.1 46.2 30.0 32±2 29.2 12±2 9.2 29 1.07 60.9 56.9 30.2 28±2 26.2 12±1 11.2 30 1.53 160 104.5 19.9 18±1 11.8 14±2 9.2 31 1.69 57.2 33.8 21.6 50±5 29.8 11±1 6.5 32 4.60 45.4 9.9 6.5 30±2 6.5 13±2 2.8 aGround-state absorption cross-section. bTriplet excited-state absorption cross section deduced from the TA spectrum. cSinglet excited-state absorption cross sections obtained from fitting the Z-scan data. dTriplet excited-state absorption cross sections obtained from fitting the Z-scan data. Data in columns 2-5 are from Ref.[56]; while data in columns 6-9 are from Ref.[63], copyright © American Chemical Society and Old City Publishing, Inc., respectively Table 5. Singlet excited-state absorption cross sections of 28 at different wavelengthsa
λ/nm σ0/10−18 cm2 σs/10−18 cm2 σs/σ0 500 9.18 17.5±0.5 1.9 532 1.30 38±2 29.2 550 0.709 27±1 38.1 570 0.302 15±2 49.7 600 0.096 25±2 260.4 aObtained from the best-fit of ps Z-scan data, reported in Ref.[63]. Copyright © Old City Publishing, Inc -
Although many of the Pt(Ⅱ) terpyridine complexes exhibited broad and strong excited-state absorption extending from the visible to the NIR regions, the lack of ground-state absorption in the longer visible and NIR regions limited their applications as broadband optical limiting materials. To solve this problem, two approaches were employed. One of the approaches was to introduce stronger electron-donating groups to the acetylide ligand to increase the energy of the highest occupied molecular orbital (HOMO) or introducing electron-withdrawing substituents to the terpyridine ligand to lower the energy of the LUMO. Either of them would lower the 1MLCT/1LLCT transition energies and shift the low-energy absorption band to longer visible spectral regions. However, the red-shifted ground-state absorption spectra increased the ground-state absorption cross sections (σ0), which significantly decreased the σex/σ0 ratios and reduced the optical limiting performances at 532 nm. Another possible approach is to incorporate two-photon absorbing unit to the complexes and utilize the two-photon induced excited-state absorption to broaden the nonlinear absorption windows to the NIR regions.
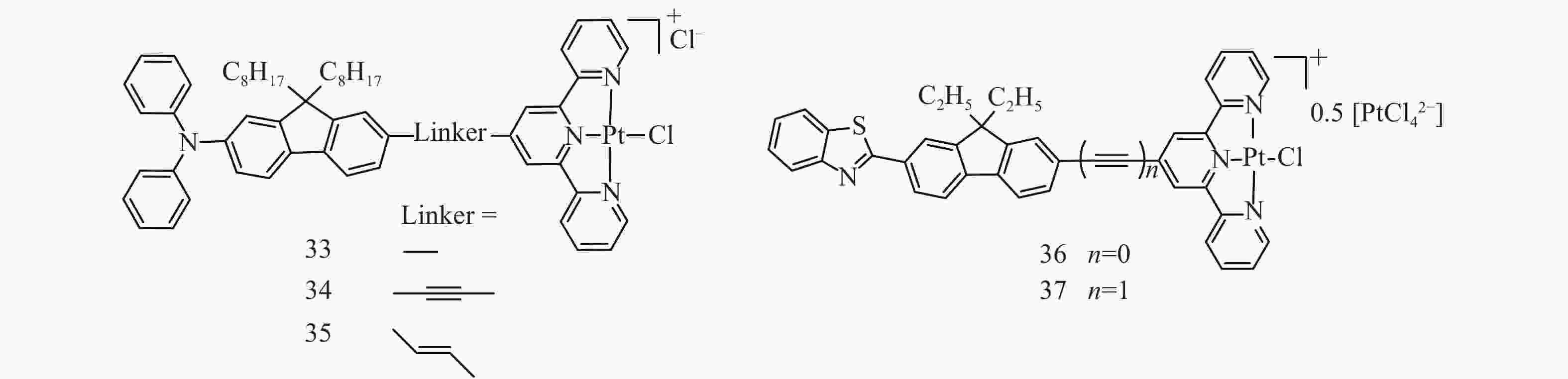
Our group reported three Pt(Ⅱ) chloride complexes 33-35 (Fig.9) bearing dipolar D-π-A terpyridine ligands, and investigated their photophysics and nonlinear absorp-tion characteristics[67]. Electron-donating 7-diphenylaminofluoren-2-yl motif was introduced to the terpyridine ligand via single, triple, or double bond in order to induce intraligand charge transfer in these complexes to increase their TPA cross sections (σ2). The effect of the different linkers was evaluated as well. As shown in Fig.10, all three complexes exhibited red-shifted strong 1π,π*/1ILCT/1MLCT absorption bands at 400-600 nm, and they gave broad excited-state absorption in the red to the NIR regions measured with the fs TA. Picosecond Z-scan measurements and the subsequent fitting with a five-level model gave the σs/σ0 ratios of 2.0-131 for 33, 2.6-78 for 34, and 1.7-21 for 35 in the wavelength range of 575-670 nm (Tab.6). At wavelengths longer than 700 nm, the observed nonlinear absorption was attributed to TPA induced excited-state absorption. The σ2 value(s) was 850 GM for 33 at 740 nm, and were 600-2000 GM at 740-825 nm for 34. The TPA of 35 was too weak to be observed. Complex 34 with the ethynylene-linker showed much stronger TPA than complexes 33 and 35 with the single bond or vinylene-linker, likely due to the better conjugation provided by the ethynylene bridge between the terpyridine ligand and the diphenylaminofluorenyl motif.
Table 6. Excited-state absorption and two-photon absorption cross sections for 33-35 at different wavelengths in CH3CN
Complex λ/nm σ0 /cm2a σS /10−18 cm2b σS/σ0 σ2 /GM 33 575 10.1 20±1 2.0 600 3.83 20±2 5.2 630 0.956 17±1 18 670 0.191 25±1 131 740 24.4c 850±50 34 550 14.7 38±2 2.6 575 6.31 24±2 3.8 600 2.49 24±2 9.6 630 0.765 26±2 34 680 0.153 12±1 78 740 7.7c 1 200±100 760 11.1c 1 000±200 800 7.7c 2 000±200 825 11.6c 600±100 35 575 25.8 43±5d 1.7 600 10.9 36±2 3.3 630 3.63 20±2 5.5 670 0.765 16±1 21 aGround-state absorption cross sections. bEffective singlet excited-state absorption cross sections with the assumption of σS2 = σS. cEstimated from the fs TA data at zero time delay. dσS2 = (12±7)×10−18 cm2. This table is modified from Ref.[67] with permission, copyright © Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 
Figure 10. (a) UV-vis absorption spectra in CH3CN for 33-35, (b) time-resolved fs transient difference absorption spectra of 33 in CH3CN, (c) open-aperture Z-scan experimental data and fitting curve for 34 in CH3CN at 740 nm. The energy used for the experiment was 6.6 μJ, and the beam waist at the focal point was 31 μm. Figures are modified from Ref.[67] with permission, copyright © Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Although the introduction of strong electron-donating diphenylamino substituent to the terpyridine ligand induced moderate TPA in the NIR regions in complexes 33-35, the strong intramolecular charge transfer character shortened the triplet excited-state lifetimes so that the transient absorption in the ns time scale was unable to be observed. In addition, the strong ground-state absorption of these three complexes at 400-600 nm made them unsuitable for optical limiting in this spectral region. To retain the TPA in these Pt(Ⅱ) complexes while shifting the major absorption bands to below 450 nm, two Pt(Ⅱ) chloride complexes bearing 7-(benzothiazol-2′-yl)-9,9-diethylfluoren-2-yl substituted terpyridine ligands (36 and 37 in Fig.9) were synthesized and studied by our group[72]. The 7-(benzothiazol-2′-yl)-9,9-diethylfluoren-2-yl substituent was attached to the 4'-position of the central pyridine ring of the terpyridine ligand via a single or triple bond linker. The band maxima of the low-energy absorption bands appeared at 428 nm and 433 nm for 36 and 37, respectively (Fig.11(a)); while the triplet excited-state absorption was broad and strong in the spectral regions of 450-820 nm and maximized at 530 and 545 nm for 36 and 37, respectively (Fig.11(b)). Meanwhile, the triplet excited states in these two complexes were long-lived (1.72-3.37 μs) and the triplet quantum yields were high (0.58-0.72, Tab.7). Therefore, both complexes exhibited pronounced optical limiting at 532 nm for ns laser pulses. With a linear transmission of 95% in a 2 mm cuvette at 532 nm, the transmission decreased to <50% at an incident fluence of ~1.8 J/cm2 (Fig.11(c)). Fitting of the Z-scan experimental data using the five-level model revealed that both complexes exhibited large ratios of the excited-state absorption cross sections vs. the ground-state absorption cross sections, with σs/σ0 of 5.35-2 237 and σT/σ0 of 5.35-3026 for 36 at 480-630 nm, and σs/σ0 of 31.8-3333 and σT/σ0 of 10.6-1786 for 37 at 500-740 nm; and TPA cross sections of 50-200 GM for 36 at 600-900 nm and 400-3700 GM for 37 at 630-910 nm. It was noted that the σs/σ0 and σT/σ0 ratios of 36 are much larger than the corresponding ratios for 37 at most of the studied wavelengths due to the weaker ground-state absorption cross sections of 36 than those of 37 at each of the corresponding wavelength (Fig.11(d) and Tab.8). In contrast, the TPA cross sections of 37 are much larger than those of 36 at each of the corresponding wavelength due to the extended π-conjugation in 37 associated with the triple bond linker. The presence of RSA in the visible spectral regions and TPA initiated excited-state absorption in the NIR regions made these two complexes promising broadband optical limiting materials.
Table 7. Photophysical parameters of 36 and 37a
λabs/nm (ε/L·mol−1·cm−1) b λS1-Sn/nm (τS/ps) b λT1-Tn/nm (ε/L·mol−1·cm−1; τT/μs; ΦT) c 36 340 (32550), 378 (20200), 428 (26900) 542 (49.4±18.3) 530 (48560; 3.37; 0.72) 37 345 (39140), 385 (sh. 20140), 433 (27040) 555 (58.7±25.4) 545 (46150; 1.72; 0.58) aThis table is modified from Ref.[72] with permission, copyright © American Chemical Society. bIn DMSO. cIn CH3CN Table 8. Absorption cross sections of 36 and 37 at selected wavelengths determined by fitting of Z-scan data
λ/nm σ0(λ)a/10−18 cm2 σS(λ)/10−18 cm2 σT(λ)c/10−18 cm2 σS/σ0 σT/σ0 σ2(λ)/GM 36 37 36 37 36 37 36 37 36 37 36 37 480 5.23 − 28 − 28 − 5.35 − 5.35 − − − 500 1.41 1.32 22 42 40 14 15.6 31.8 28.4 10.6 − − 532 0.0955 0.390 42 19 68 21 440 48.7 712 53.8 − − 550 0.0435 0.187 35 35 66 28 805 187 1517 150 − − 600 0.0222 0.0726 21b 40 29 15 946 551 1306 207 50 − 630 0.0076 0.0336 17b 29b 23 13 2237 863 3026 387 110 1500 680 ~0 0.0153 19b 27b 23 13 −1765 − 850 160 600 740 ~0 0.0084 22b 28b 31 15 −3333 −1786 65 550 760 ~0 ~0 22b 29b 36 16 − − − − 90 400 800 ~0 ~0 22b 23b 53 20 − − − − 60 450 825 ~0 ~0 − 43b − 21 − − − − 200d 500 850 ~0 ~0 − − − − − − − − 280d 3700e 875 ~0 ~0 − − − − − − − − 180d 3000e 900 ~0 − − − − − − − − − 200d − 910 ~0 ~0 − − − − − − − − − 1700e a Deduced from UV-Vis absorption spectrum. b Estimated from σS(532 nm) and the femtosecond transient difference absorption spectrum at zero time delay. These values are effective cross sections for the singlet excited states because the fs TA includes contributions from both S1 and S2 states. c σT(532 nm) was determined from the combined fitting of nanosecond and picosecond Z-scan data. For other wavelengths, σT(λ) was estimated from σT(532 nm) and the femtosecond transient difference absorption spectrum at 5.9 ns time delay. d Effective TPA cross sections for excited-state-assisted TPA. e Effective TPA cross section for the Z-scan of lowest energy (11.5 µJ at 825 nm, 7.9 µJ at 850 nm, 8.3 µJ at 875 nm, and 10.0 µJ at 900 nm). This table is modified from Ref.[72] with permission, copyright © American Chemical Society 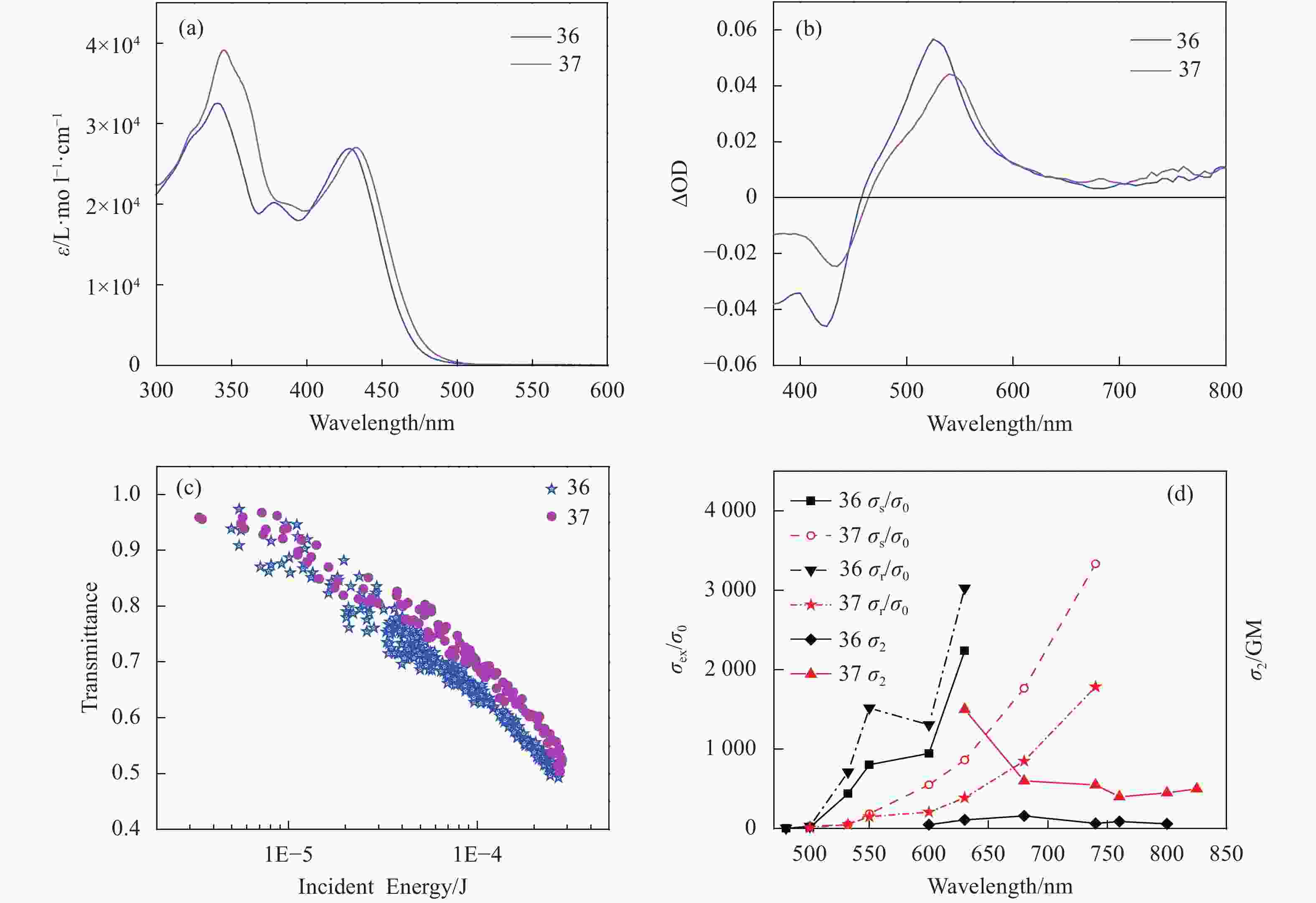
Figure 11. (a) UV-vis absorption spectra of 36 and 37 in DMSO, (b) nanosecond TA spectra of 36 and 37 in CH3CN immediately after laser excitation. λex = 355 nm. A355 = 0.4 in a 1 cm cuvette. (c) optical limiting curves of 36 and 37 in DMSO solution for 4.1 ns laser pulses at 532 nm. The linear transmission of the solution was adjusted to 95% in a 2 mm cuvette. The beam waist at the focal plane was 72 μm, (d) wavelength dispersion of the ratios of excited-state absorption cross section to that of the ground-state absorption (σex/σ0) and TPA cross section (σ2) for 36 and 37 in DMSO solution. Figures are modified from Ref.[72] with permission, copyright © American Chemical Society
Very recently, Yam’s group reported the TPA cross sections of two 1,3,5-triethynylbenzene-based alkynylplatinum(Ⅱ) terpyridine complexes (38 and 39 in Fig.12) that were determined by two-photon induced fluo-rescence measurement[76]. The σ2 values were reported to be 2 and 8 GM at 720 nm for 38 and 39, respectively. The very small σ2 values of this type of complexes could be attributed to the limited π-framework of the ligands in these complexes.
To improve the TPA of the alkynylplatinum(Ⅱ) terpyridine complexes, Yam’s group reported a series of truxene-containing mononuclear or multinuclear alkynylplatinum(Ⅱ) terpyridine complexes 40-44 (Fig.12)[77]. The σ2 values of 40 and 44 at 720 nm were measured by two-photon induced fluorescence and reported to be 37 and 108 GM, respectively. An obviously increased σ2 value was achieved in 40 compared to that of 38, and the increased σ2 value of 44 with respect to that of 40 should be ascribed to the increased number of Pt(Ⅱ) terpyridine moieties.
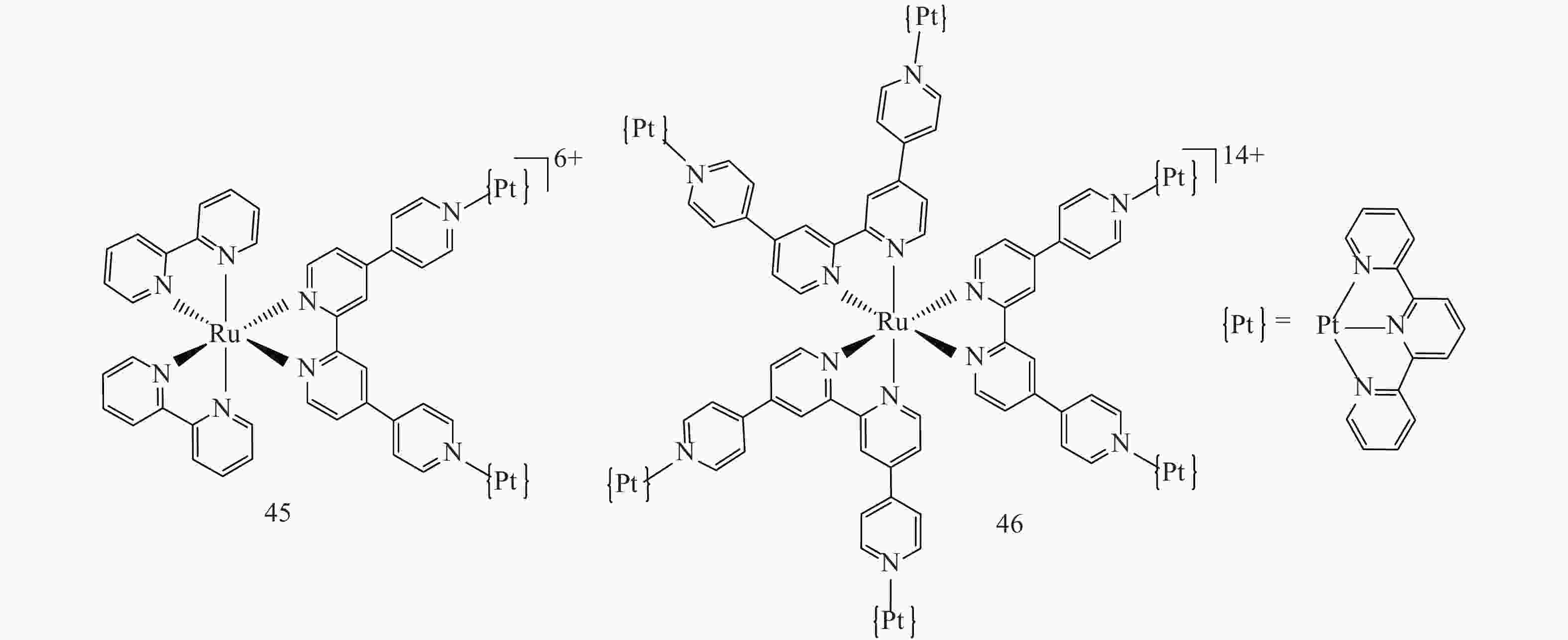
Shi/Coe and co-workers reported the TPA of a trinuclear RuPt2 and a heptanuclear RuPt6 complexes by attaching PtⅡ 2,2′:6′,2″-terpyridine (tpy) moieties to RuⅡ 4,4′:2′,2″:4″,4‴-quaterpyridine (qpy) complexes (45 and 46 in Fig.13)[73]. Z-scan measurement revealed higher TPA cross sections from these multinuclear complexes, with a maximal σ2 value of 301 GM at 834 nm for RuPt2 (45) and 523 GM at 850 nm for RuPt6 (46). Attaching PtⅡ(tpy) moieties tripled or quadrupled the TPA cross sections of these multinuclear complexes when compared to the RuⅡ-based core. However, the electron-normalized maximal σ2 values (σ2/Neff, where Neff is the total number of π-bonding electrons in the complex (excluding the metal d-electrons)) were 0.043 and0.016 GM for 45 and 46, respectively, indicating no electronic interactions between the metal centers and thus no synergistic enhancement effects.
-
The reported work on the nonlinear absorption of Pt(Ⅱ) terpyridine complexes revealed that generally these complexes possessed weak 1MLCT/1LLCT ground-state absorption in the 400-600 nm spectral region, while the excited-state absorption of these complexes were broad and moderately strong at 500-800 nm. The 1MLCT/1LLCT absorption band(s) and the excited-state absorption can be readily tuned by electron-donating or withdrawing substituents at the acetylide or the terpyridine ligand. Specially, introducing electron-donating substituentto the acetylide or terpyridine ligand, or improving the coplanarity between the aromatic substituent and the terdentate core ligand could red-shift the 1MLCT/1LLCT absorption band(s). Unfortunately, strong electron-donating substituents significantly reduced the triplet excited-state lifetime and consequently decreased/quenched the triplet excited-state absorption. Meanwhile, the red-shifted 1MLCT/1LLCT band(s) increased the ground-state absorption cross section at 532 nm and consequently reduced the RSA and optical limiting at532 nm due to the reduced ratio of σex/σ0. The TPA cross sections (σ2) of the Pt(Ⅱ) terpyridine complexes bearing small π-conjugated ligands were typically small. However, the σ2 values could be dramatically improved by extending the π-conjugation on the terpyridine ligand. Particularly, incorporation of π-conjugated aromatic substituent without strong electron-donating ability could restrain the lowest-energy ground-state absorption band to <500 nm while keeping a long-lived triplet excited state with broadband excited-state absorption, and moderately strong TPA in the NIR regions. This approach could provide a solution for developing broadband optical limiting materials.
-
The author thanks the National Science Foundation (CHE 0449598) and the US Army Research Laboratory (W911NF 06-2-0032 and W911NF 10-2-0055) for financialsupport.
Nonlinear absorption and optical limiting of platinum(Ⅱ) terpyridine complexes (Invited)
-
摘要: 本文总结了2003~2019年报道的联三吡啶铂(Ⅱ)配合物的反饱和吸收或双光子吸收及光限幅研究进展,并对这类配合物的光物理特性,包括基态吸收ˎ激发态吸收ˎ激发态寿命和三重态量子产率ˎ在532 nm的反饱和吸收或光限幅,在近红外光区的双光子吸收,以及构效关系进行了评估。首先介绍了目前光限幅材料和器件的研究现状,对反饱和吸收和双光子吸收材料的基本要求,以及平面正方形铂(Ⅱ)配合物的种类和特性;其次讨论了六个系列联三吡啶类铂(Ⅱ)配合物的反饱和吸收或光限幅及构效关系;随后总结了五个系列联三吡啶铂(Ⅱ)配合物的双光子吸收及结构变化对双光子吸收截面的影响;最后对文献报道的工作进行了小结。根据文献报道的工作发现的一个趋势是:在联三吡啶配体或单齿炔配体上引入取代基可以调节基态和激发态的吸收,特别是在配体上引入给电子基团或增加联三吡啶和其上的芳香环取代基的共平面性会引起基态吸收红移但降低或淬灭激发态的吸收,这样会降低在532 nm的反饱和吸收或光限幅。但是扩展联三吡啶配体上的共轭体系则能大幅提高铂(Ⅱ)配合物的双光子吸收截面,尤其是在联三吡啶配体上引入吸电子的共轭芳香环取代基可以控制基态吸收在500 nm以下,同时保持了在可见光区的长寿命宽幅三重激发态吸收和在近红外光区的中等强度的双光子吸收,这对研制宽幅激光限幅材料有重要意义。Abstract: The reported work in 2003-2019 on the reverse saturable absorption (RSA) or two-photon absorption (TPA) and/or optical limiting (OPL) of platinum(II) terpyridine complexes was summarized in this minireview. Photophysical properties, including the ground-state absorption (GSA), excited-state absorption (ESA), excited-state lifetimes, and the quantum yields of triplet excited-state formation, RSA/OPL at 532 nm for ns laser pulses, TPA characteristics in the near-IR spectral regions, and the structure-property correlations were reviewed. This paper is composed of four sections. First, the current status of OPL materials and devices, the general requirements for reverse saturable absorbers and two-photon absorbing materials, and the different types and characteristics of square-planar platinum(II) complexes were briefly introduced. Then the photophysics and RSA/OPL of six series of Pt(Ⅱ) terpyridine-analogous complexes and the structure-property correlations were discussed. Following it the TPA of five series of Pt(Ⅱ) terpyridine complexes and the impacts of structural variations on the TPA cross sections (σ2) were reviewed. Finally, brief conclusions were drawn based on the reported studies. A general trend discovered was that the charge transfer absorption band(s) and the ESA can be readily tuned by substituents on the acetylide or the terpyridine ligand. Introducing electron-donating substituent to the acetylide or terpyridine ligand or improving the coplanarity between the aromatic substituent and the terpyridine ligand red-shifted the ground-state charge-transfer absorption band(s) at the price of decreasing/quenching the triplet ESA, which consequently reduced the RSA/OPL at 532 nm. Extending the π-conjugation of the terpyridine ligand dramatically improved the σ2 values of the Pt(Ⅱ) terpyridine complexes. Incorporation of electron-withdrawing π-conjugated aromatic substituent restrained the GSA to < 500 nm while keeping a long-lived triplet excited state with broadband ESA in the visible spectral regions and moderately strong TPA in the NIR regions. This approach could provide a solution for developing broadband OPL materials.
-
Figure 3. (a) Linear absorption spectra of complexes 1-3 (8.8×10−5 mol/L) in CH2Cl2/CH3OH (8∶2) in a 2 mm cell, (b) triplet transient difference absorption (TA) spectra of 2 (2.39×10−5 mol/L) and 3 (2.09×10−5 mol/L) in a 1-cm cell. The TA spectrum of 1 was similar to that of 2, thus was not shown in Fig.3(b), (c) Z-scan experimental data and fitting curve for 3 in CH2Cl2/CH3OH (8∶2) with a concentration of 3×10−4 mol/L and a linear transmission of 95% at 532 nm in a 1 mm cell. Figures a and b are modified from Ref. [50] with permission, copyright © American Institute of Physics
Figure 4. (a) and (b) Linear absorption spectra of complexes 4-12 (1×10−5 mol/L) in acetonitrile in a 1 cm cuvette, (c) ns TA spectra of 4, 5, 6 and 10 in acetonitrile in a 1 cm cell (A = 0.4 at 355 nm). See Ref. [51] for the ns TA spectra of 11 and 12 in acetonitrile, (d) time-resolved fs TA spectra of 11 in acetonitrile, (e) optical limiting curves of 4, 5, 10, 11 and 12 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% in the 2 mm cell. Figures are modified from Refs. [51] and [52] with permission, copyright © Chinese Optical Society and American Chemical Society, respectively
Figure 5. (a) Linear absorption spectra of 19-22 (2.0×10−5 mol/L) in acetonitrile in a 1 cm cuvette, (b) ns TA spectra of 19-22 in acetonitrile in a 1 cm cell (A = 0.4 at 355 nm), (c) optical limiting curves of 19-22 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% at 532 nm in the 2 mm cell. Figures are modified from Ref. [53] with permission, copyright © American Chemical Society
Figure 7. (a) Linear absorption spectra of 23-27 in CH2Cl2, (b) ns time-resolved TA spectra of 25 in CH2Cl2 in a 1 cm cell (6.2×10−5 mol/L (A355 = 0.888)), (c) ns time-resolved TA spectra of 26 in CH2Cl2 in a 1 cm cell (3.9×10−5 mol/L (A355 = 0.564)), (d) optical limiting curves of 25-27 in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 70% at 532 nm in the 2 mm cell. 25 and 26 were dissolved in CH2Cl2, and 27 was dissolved in DMF. Figures are modified from Ref. [5] with permission, copyright © American Chemical Society
Figure 8. (a) Linear absorption spectra of 28-32 in acetonitrile, (b) ns TA spectra of 28-32 in acetonitrile in a 1 cm cell (λex = 355 nm), (c) optical limiting curves of 28-32 in acetonitrile in a 2 mm cell at 532 nm for 4.1 ns laser pulses. The linear transmission for all solutions was adjusted to 75% at 532 nm in the 2 mm cell. Figures are modified from Ref.[56] with permission, copyright © American Chemical Society
Figure 10. (a) UV-vis absorption spectra in CH3CN for 33-35, (b) time-resolved fs transient difference absorption spectra of 33 in CH3CN, (c) open-aperture Z-scan experimental data and fitting curve for 34 in CH3CN at 740 nm. The energy used for the experiment was 6.6 μJ, and the beam waist at the focal point was 31 μm. Figures are modified from Ref.[67] with permission, copyright © Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Figure 11. (a) UV-vis absorption spectra of 36 and 37 in DMSO, (b) nanosecond TA spectra of 36 and 37 in CH3CN immediately after laser excitation. λex = 355 nm. A355 = 0.4 in a 1 cm cuvette. (c) optical limiting curves of 36 and 37 in DMSO solution for 4.1 ns laser pulses at 532 nm. The linear transmission of the solution was adjusted to 95% in a 2 mm cuvette. The beam waist at the focal plane was 72 μm, (d) wavelength dispersion of the ratios of excited-state absorption cross section to that of the ground-state absorption (σex/σ0) and TPA cross section (σ2) for 36 and 37 in DMSO solution. Figures are modified from Ref.[72] with permission, copyright © American Chemical Society
Table 1. Optical limiting parameters at 532 nm for ns laser pulsesa
Complexes Fth/J·cm−2 Fthroughput/J·cm−2 Tlim(at 3.6 J·cm−2) 1 0.03 1.07 0.30 2 0.03 1.16 0.32 3 0.02 0.45 0.12 aModified from Ref. [50] with permission. Copyright © American Institute of Physics Table 2. Photophysical parameters and optical limiting data for 4, 5, 10, 11, 12, and 19-22 in acetonitrile[51,53]
Complexes τT/ns σ0 c/10−19 cm2 Fth d/mJ·cm-2 Tlime σeff/σ0 4 766a 2.43 48 0.28f, 0.25g >3.57f, >3.89g 5 659a 7.18 144 0.34f >3.02f 10 672a 11.2 1090 0.44f >2.30f 11 62a 2.50[57] 62 0.19f >4.66f 12 51a 2.40 900 0.45f >2.24f 19 255b 18.1 250 0.27g >3.67g 20 408b 4.30 370 0.27g >3.67g 21 384b 17.6 490 0.32g >3.19g 22 2540b 13.4 52 0.18g >4.81g aTriplet excited-state lifetime deduced from the decay of the TA at 700 nm, from Ref.[51]. bTriplet excited-state lifetime deduced from the decay of the TA at 680 nm, from Ref.[53]. cGround-state absorption cross section at 532 nm, from Refs. [51], [53] or [57]. dRSA threshold when the transmission dropped to 90% of the linear transmission. eNonlinear transmittance at high incident fluence. fAt incident fluence of 2.5 J/cm2. gAt incident fluence of 3.0 J/cm2. This table is modified from Refs.[51] and [53] with permission. Copyrights © Chinese Optical Society and American Chemical Society, respectively Table 3. Photophysical parameters of 28-32 in CH3CN
28 29 30 31 32 λabs/nma 463 436 456 463 470 λS1-Sn/nmb 717 685 712 719 766 τs/psc 37±23 56±17 139±128 42±8 46±16 λT1-Tn/nmd 725 720 755 730 785 τT/nse 420 660 130 340 130 ΦTf 0.65 0.53 0.19 0.64 0.66 τisc/psg 57 106 732 66 70 a 1MLCT/1LLCT band maxima. bFemtosecond (fs) TA band maxima. cSinglet excited-state lifetimes. dNanosecond (ns) TA band maxima. eTriplet excited-state lifetimes. fQuantum yields of the triplet excited-state formation. gIntersystem crossing (ISC) time. Data in rows 2 and 5-7 are from Ref. 56; data in rows 3, 4 and 8 are from Ref.[63], copyright © American Chemical Society and Old City Publishing, Inc., respectively Table 4. Ground-state and excited-state absorption cross sections of 28-32 in CH3CN at 532 nm
σ0a/10−18 cm2 σTb/10−18 cm2 σT/σ0 σTΦT/σ0 σsc/10−18 cm2 σs/σ0 σTd/10−17 cm2 σT/σ0 28 1.30 60.1 46.2 30.0 32±2 29.2 12±2 9.2 29 1.07 60.9 56.9 30.2 28±2 26.2 12±1 11.2 30 1.53 160 104.5 19.9 18±1 11.8 14±2 9.2 31 1.69 57.2 33.8 21.6 50±5 29.8 11±1 6.5 32 4.60 45.4 9.9 6.5 30±2 6.5 13±2 2.8 aGround-state absorption cross-section. bTriplet excited-state absorption cross section deduced from the TA spectrum. cSinglet excited-state absorption cross sections obtained from fitting the Z-scan data. dTriplet excited-state absorption cross sections obtained from fitting the Z-scan data. Data in columns 2-5 are from Ref.[56]; while data in columns 6-9 are from Ref.[63], copyright © American Chemical Society and Old City Publishing, Inc., respectively Table 5. Singlet excited-state absorption cross sections of 28 at different wavelengthsa
λ/nm σ0/10−18 cm2 σs/10−18 cm2 σs/σ0 500 9.18 17.5±0.5 1.9 532 1.30 38±2 29.2 550 0.709 27±1 38.1 570 0.302 15±2 49.7 600 0.096 25±2 260.4 aObtained from the best-fit of ps Z-scan data, reported in Ref.[63]. Copyright © Old City Publishing, Inc Table 6. Excited-state absorption and two-photon absorption cross sections for 33-35 at different wavelengths in CH3CN
Complex λ/nm σ0 /cm2a σS /10−18 cm2b σS/σ0 σ2 /GM 33 575 10.1 20±1 2.0 600 3.83 20±2 5.2 630 0.956 17±1 18 670 0.191 25±1 131 740 24.4c 850±50 34 550 14.7 38±2 2.6 575 6.31 24±2 3.8 600 2.49 24±2 9.6 630 0.765 26±2 34 680 0.153 12±1 78 740 7.7c 1 200±100 760 11.1c 1 000±200 800 7.7c 2 000±200 825 11.6c 600±100 35 575 25.8 43±5d 1.7 600 10.9 36±2 3.3 630 3.63 20±2 5.5 670 0.765 16±1 21 aGround-state absorption cross sections. bEffective singlet excited-state absorption cross sections with the assumption of σS2 = σS. cEstimated from the fs TA data at zero time delay. dσS2 = (12±7)×10−18 cm2. This table is modified from Ref.[67] with permission, copyright © Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Table 7. Photophysical parameters of 36 and 37a
λabs/nm (ε/L·mol−1·cm−1) b λS1-Sn/nm (τS/ps) b λT1-Tn/nm (ε/L·mol−1·cm−1; τT/μs; ΦT) c 36 340 (32550), 378 (20200), 428 (26900) 542 (49.4±18.3) 530 (48560; 3.37; 0.72) 37 345 (39140), 385 (sh. 20140), 433 (27040) 555 (58.7±25.4) 545 (46150; 1.72; 0.58) aThis table is modified from Ref.[72] with permission, copyright © American Chemical Society. bIn DMSO. cIn CH3CN Table 8. Absorption cross sections of 36 and 37 at selected wavelengths determined by fitting of Z-scan data
λ/nm σ0(λ)a/10−18 cm2 σS(λ)/10−18 cm2 σT(λ)c/10−18 cm2 σS/σ0 σT/σ0 σ2(λ)/GM 36 37 36 37 36 37 36 37 36 37 36 37 480 5.23 − 28 − 28 − 5.35 − 5.35 − − − 500 1.41 1.32 22 42 40 14 15.6 31.8 28.4 10.6 − − 532 0.0955 0.390 42 19 68 21 440 48.7 712 53.8 − − 550 0.0435 0.187 35 35 66 28 805 187 1517 150 − − 600 0.0222 0.0726 21b 40 29 15 946 551 1306 207 50 − 630 0.0076 0.0336 17b 29b 23 13 2237 863 3026 387 110 1500 680 ~0 0.0153 19b 27b 23 13 −1765 − 850 160 600 740 ~0 0.0084 22b 28b 31 15 −3333 −1786 65 550 760 ~0 ~0 22b 29b 36 16 − − − − 90 400 800 ~0 ~0 22b 23b 53 20 − − − − 60 450 825 ~0 ~0 − 43b − 21 − − − − 200d 500 850 ~0 ~0 − − − − − − − − 280d 3700e 875 ~0 ~0 − − − − − − − − 180d 3000e 900 ~0 − − − − − − − − − 200d − 910 ~0 ~0 − − − − − − − − − 1700e a Deduced from UV-Vis absorption spectrum. b Estimated from σS(532 nm) and the femtosecond transient difference absorption spectrum at zero time delay. These values are effective cross sections for the singlet excited states because the fs TA includes contributions from both S1 and S2 states. c σT(532 nm) was determined from the combined fitting of nanosecond and picosecond Z-scan data. For other wavelengths, σT(λ) was estimated from σT(532 nm) and the femtosecond transient difference absorption spectrum at 5.9 ns time delay. d Effective TPA cross sections for excited-state-assisted TPA. e Effective TPA cross section for the Z-scan of lowest energy (11.5 µJ at 825 nm, 7.9 µJ at 850 nm, 8.3 µJ at 875 nm, and 10.0 µJ at 900 nm). This table is modified from Ref.[72] with permission, copyright © American Chemical Society -
[1] Miller M J, Mott A G, Ketchel B P. General optical limiting requirements [C]//SPIE Conference on Nonlinear Optical Liquids for Power Limiting and Imaging, 1988, 3472: 24-29. [2] Scott W B. Southwest pilot injured by laser [J]. Aviation Week and Space Technology, Nov. 20, 1995: 92. [3] Gertz B. Russians fire laser at helicopter [N]. The Washington Times, 1997-05-14(A1). [4] Pentagon is unable to link Russian laser to eye injury [N]. The Washington Post, 1997-06-27(A22). [5] Bunning T J, Natarajan L V, Schmitt M G, et al. Optical limiting in solutions of diphenyl polyenes [J]. Applied Optics, 1991, 30(30): 4341-4349. [6] Soileau M J. Materials for Optical Switches, Isolators, and Limiters, Proc SPIE, vol. 1105 [C]. US: SPIE Press, 1989. [7] Shirk J S, Pong R G S, Bartoli F J, et al. Optical limiter using a lead phthalocyanine [J]. Applied Physics Letters, 1993, 63(14): 1880-1882. [8] Soileau M J. Nonlinears Optical Materials for Switching and Limiting, Proc SPIE, vol. 2229 [C]. US: SPIE Press, 1994. [9] Tutt L W, Kost A. Optical limiting performance of C60 and C70 solutions [J]. Nature, 1992, 356: 225-226. [10] Lawson C M. Nonlinear Optical Liquids and Power Limiters, Proc SPIE, vol. 3146 [C]. US: SPIE Press, 1997. [11] Lawson C M. Nonlinear Optical Liquids for Power Limiting and Imaging, Proc SPIE, vol. 3472 [C]. US: SPIE Press, 1998. [12] Lawson C M. Power-Limiting Materials and Devices, Proc SPIE, vol. 3798 [C]. US: SPIE Press, 1999. [13] Eich M, Kuzyk M G, Lawson C M, et al. Linear, Nonlinear, and Power-Limiting Organics, Proc SPIE, vol. 4106 [C]. US: SPIE Press, 2000. [14] Crane R, Lewis K, Van Stryland E, et al. Materials for optical limiting, MRS Proceedings, vol. 374 [C]. US: Materials Research Society Symposium, 1994. [15] Sutherland R, Pachter R, Hood P, et al. Materials for optical limiting Ⅱ, MRS Proceedings, vol. 479 [C]. US: Materials Research Society Symposium, 1997. [16] Nashimoto K, Pachter R, Wessels B W, et al. Thin films for optical waveguide devices and materials for optical limiting, MRS Proceedings, vol. 597 [C]. US: Materials Research Society Symposium, 2000. [17] Li C, Zhang L, Wang R, et al. Dynamics of reverse saturable absorption and all-optical switching in C60 [J]. Journal of the Optical Society of America B, 1994, 11(8): 1356-1360. [18] Perry J W, Mansour K, Marder S R, et al. Enhanced reverse saturable absorption and optical limiting in heavy-atom-substituted phthalocyanines [J]. Optics Letters, 1994, 19(9): 625-627. [19] Penzkofer A. Passive Q-switching and mode-locking for the generation of nanosecond to femtosecond pulses [J]. Applied Physics B, 1988, 46(1): 43-60. [20] Reddy K P J. Applications of reverse saturable absorbers in laser science [J]. Current Issue, 1991, 61(8): 520-525. [21] Speiser S, Orenstein M. Spatial light modulation via optically induced absorption changes in molecules [J]. Applied Optics, 1988, 27(14): 2944-2948. [22] Yehuda B B, Harter D J, Raanan B. Optical pulse compressor composed of saturable and reverse saturable absorbers [J]. Chemical Physics Letter, 1986, 126(3-4): 280-284. [23] Shen Y, Shuhendler A J, Ye D, et al. Two-photon excitation nanoparticles for photodynamic therapy [J]. Chemical Society Reviews, 2016, 45(24): 6725-6741. [24] Gareth W J A. Photochemistry and Photophysics of Coordination Compounds: Platinum [M]//Vincenzo Balzani Sebastiano Campagna. Photochemistry and Photophysics of Coordination Compounds Ⅱ. Berlin: Springer, 2007: 205-268. [25] Eryazici I, Moorefield C N, Newkome G R. Square-planar Pd(Ⅱ), Pt(Ⅱ), and Au(Ⅲ) terpyridine complexes: Their syntheses, physical properties, supramolecular constructs, and biomedical activities [J]. Chemical Reviews, 2008, 108(6): 1834-1895. [26] Lippard S J. Platinum complexes: probes of polynucleotide structure and antitumor drugs [J]. Accounts of Chemical Research, 1978, 11(5): 211-217. [27] Ratilla E M A, Brothers H M, Kostic N M. A transition-metal chromophore as a new, sensitive spectroscopic tag for proteins. Selective covalent labeling of histidine residues in cytochromesc with chloro(2,2':6'2"-terpyridine)platinum(Ⅱ) chloride [J]. Journal of the American Chemical Society, 1987, 109(15): 4592-4599. [28] Wong K M-C, Tang W-S, Lu X, et al. Functionalized platinum(Ⅱ) terpyridyl alkynyl complexes as colorimetric and luminescence pH sensors [J]. Inorganic Chemistry, 2005, 44(5): 1492-1498. [29] Wadas T J, Chakraborty S, Lachicotte R J, et al. Facile synthesis, structure, and luminescence properties of Pt(diimine)bis(arylacetylide) chromophore-donor dyads [J]. Inorganic Chemistry, 2005, 44(8): 2628-2638. [30] Lu W, Mi B, Chan M C W, et al. Light-emitting tridentate cyclometalated platinum(Ⅱ) complexes containing σ-alkynyl auxiliaries: Tuning of photo- and electrophosphorescence [J]. Journal of the American Chemical Society, 2004, 126(15): 4958-4971. [31] Fort Y, Comoy C. NHC—Nickel and —Platinum complexes in catalysis[J]. RSC Catalysis Series 6, 2011: 284-316. [32] Staromlynska J, McKay T J, Bolger J A, et al. Evidence for broadband optical limiting in a Pt:ethynyl compound [J]. Journal of the Optical Society of America B, 1998, 15(6): 1731-1736. [33] McKay T J, Bolger J A, Staromlynska J, et al. Linear and nonlinear optical properties of platinum-ethynyl [J]. The Journal of Chemical Physics, 1998, 108(13): 5537-5541. [34] McKay T J, Staromlynska J, Davy J R, et al. Cross sections for excited-state absorption in a Pt:ethynyl complex [J]. Journal of the Optical Society of America B, 2001, 18(3): 358-362. [35] Price R S, Dubinina G, Wicks G, et al. Polymer monoliths containing two-photon absorbing phenylenevinylene platinum(Ⅱ) acetylide chromophores for optical power limiting [J]. ACS Applied Materials & Interfaces, 2015, 7(20): 10795-10805. [36] Yao C, Tian Z, Jin D, et al. Platinum(II) acetylide complexes with star-and V-shaped configurations possessing good trade-off between optical transparency and optical power limiting performance [J]. Journal of Materials Chemistry C, 2017, 5(34): 11672-11682. [37] Glimsdal E, Carlsson M, Kindahl T, et al. Luminescence, singlet oxygen production, and optical power limiting of some diacetylide platinum(Ⅱ) diphosphine complexes [J]. The Journal of Physical Chemistry A, 2010, 114(10): 3431-3442. [38] Cooper T M, Haley J E, Krein D M, et al. Two-photon spectroscopy of a series of platinum acetylides: Conformation-induced ground-state symmetry breaking [J]. The Journal of Physical Chemistry A, 2017, 121(29): 5442-5449. [39] Collin J-P, Harriman A, Heitz V, et al. Photoinduced electron- and energy-transfer processes occurring within porphyrin-metal-bisterpyridyl conjugates [J]. Journal of the American Chemical Society, 1994, 116(13): 5679-5690. [40] Harriman A, Odobel F, Sauvage J-P. Multistep electron transfer between porphyrin modules assembled around a ruthenium center [J]. Journal of the American Chemical Society, 1995, 117(37): 9461-9472. [41] Dixon I M, Collin J-P, Sauvage J-P, et al. Porphyrinic dyads and triads assembled around iridium(Ⅲ) bis-terpyridine: Photoinduced electron transfer processes [J]. Inorganic Chemistry, 2001, 40(22): 5507-5517. [42] Fang H, Du C, Qu S, et al. Self-assembly of the[60]fullerene-substituted oligopyridines on Au nanoparticles and the optical nonlinearities of the nanoparticles [J]. Chemical Physics Letters, 2002, 364(3-4): 290-296. [43] Newkome G R, Cardullo F, Constable E C, et al. Metallomicellanols: incorporation of ruthenium(Ⅱ)–2,2': 6',2″-terpyridine triads into cascade polymers [J]. Journal of the Chemical Society, Chemical Communications, 1993, 11: 925-927. [44] Newkome G R, He E, Godínez L A, et al. Electroactive metallomacromolecules via tetrabis(2,2':6',2"-terpyridine) ruthenium(Ⅱ) complexes: Dendritic nanonetworks toward constitutional isomers and neutral species without external counterions [J]. Journal of the American Chemical Society, 2000, 122(41): 9993-10006. [45] Cheung T-C, Cheung K-K, Peng S-M, et al. Photoluminescent cyclometallated diplatinum(Ⅱ,Ⅱ) complexes: photophysical properties and crystal structures of [PtL(PPh3)]ClO4 and[Pt2L2(μ-dppm)][ClO4]2 (HL = 6-phenyl-2,2′-bipyridine, dppm = Ph2PCH2PPh2) [J]. Journal of the Chemical Society, Dalton Transactions, 1996, 8: 1645-1651. [46] Lai S-W, Chan M C-W, Cheung T-C, et al. Probing d8-d8 interactions in luminescent mono- and binuclear cyclometalated platinum(Ⅱ) complexes of 6-phenyl-2,2'-bipyridines [J]. Inor-ganic Chemistry, 1999, 38(18): 4046-4055. [47] Lu W, Chan M C W, Cheung K-K, et al. π-π interactions in organometallic systems. crystal structures and spectroscopic properties of luminescent mono-, bi-, and trinuclear trans-cyclometalated platinum(Ⅱ) complexes derived from 2,6-diphenylpyridine [J]. Organometallics, 2001, 20(12): 2477-2486. [48] Lu W, Chan M C W, Zhu N, et al. Structural and spectroscopic studies on Pt···Pt and π-π interactions in luminescent multinuclear cyclometalated platinum(Ⅱ) homologues tethered by oligophosphine auxiliaries [J]. Journal of the American Chemical Society, 2004, 126(24): 7639-7651. [49] Wang Y, Yang Q, Wu L, et al. Synthesis and luminescent properties of an acetylide‐bridged dinuclear platinum(Ⅱ) terpyridyl complex [J]. Chinese Journal of Chemistry, 2004, 22(1): 114-116. [50] Sun W, Wu Z, Yang Q, et al. Reverse saturable absorption of platinum ter/bipyridyl polyphenylacetylide complexes [J]. Applied Physics Letters, 2003, 82(6): 850-852. [51] Sun W , Guo F. Excited state absorption and optical limiting of platinum(Ⅱ) 4'-arylterpyridyl acetylide complexes [J]. Chinese Optics Letters, 2005, 3(S): S34-S37. [52] Guo F, Sun W, Liu Y, et al. Synthesis, photophysics, and optical limiting of platinum(Ⅱ) 4'-tolylterpyridyl arylacetylide complexes [J]. Inorganic Chemistry, 2005, 44(11): 4055-4065. [53] Guo F, Sun W. Photophysics and optical limiting of platinum(Ⅱ) 4'-arylterpyridyl phenylacetylide complexes [J]. The Journal of Physical Chemistry B, 2006, 110(30): 15029-15036. [54] Sun W, Zhu H, Barron P M. Binuclear cyclometalated platinum(Ⅱ) 4,6-Diphenyl-2,2'-bipyridine complexes: Interesting photoluminescent and optical limiting materialsng materials [J]. Chemistry of Materials, 2006, 18(10): 2602-2610. [55] Shao P, Sun W. Trinuclear platinum(Ⅱ) 4,6-diphenyl-2,2'-bipyridyl complex with bis(diphenylphosphinomethyl) phenylphosphine auxiliary ligand: synthesis, structural characterization, and photophysics [J]. Inorganic Chemistry, 2007, 46(21): 8603-8612. [56] Ji Z, Li Y, Sun W. 4′-(5′′′-R-pyrimidyl)-2,2′:6′,2″-terpyridyl (R = H, OEt, Ph, Cl, CN) platinum(Ⅱ) phenylacetylide complexes: Synthesis and photophysics [J]. Inorganic Chemistry, 2008, 47(17): 7599-7607. [57] Pritchett T M, Sun W , Guo F, et al. Excited-state absorption in a terpyridyl platinum(Ⅱ) pentynyl complex [J]. Optics Letters, 2008, 33(10): 1053-1055. [58] Shao P, Li Y, Sun W. Cyclometalated platinum(Ⅱ) complex with strong and broadband nonlinear optical response [J]. The Journal of Physical Chemistry A, 2008, 112(6): 1172-1179. [59] Shao P, Li Y, Sun W. Platinum(Ⅱ) 2,4-Di(2′-pyridyl)-6-(p-tolyl)-1,3,5-triazine complexes: Synthesis and photophysics [J]. Organometallics, 2008, 27(12): 2743-2749. [60] Li Y, Pritchett T, Shao P, et al. Excited-state absorption of mono-, di- and tri-nuclear cyclometalated platinum 4,6-diphenyl-2,2′-bipyridyl complexes [J]. Journal of Organometallic Chemistry, 2009, 694(23): 3688-3691. [61] Ji Z, Azenkeng A, Hoffmann M, et al. Synthesis and photophysics of 4′-R-2,2′;6′,2″-terpyridyl (R = Cl, CN, N(CH3)2) platinum(Ⅱ) phenylacetylide complexestinum(Ⅱ) phenylacetylide complexes [J]. Dalton Trans, 2009, 37: 7725-7733. [62] Shao P, Li Y, Azenkeng A, et al. Influence of alkoxyl substituent on 4,6-diphenyl-2,2′-bipyridine ligand on photophysics of cyclo-metalated platinum(Ⅱ) complexes: Admixing intraligand charge transfer character in low-lying excited states [J]. Inorganic Chemistry, 2008, 48(6): 2407-2419. [63] Sun W, Li Y, Pritchett T, et al. Excited-state absorption of 4′-(5′′′-R-pyrimidyl)-2,2′:6′,2″-terpyridyl platinum(Ⅱ) phenylacetylide complexes [J]. Nonlinear Optics, Quantum Optics: Concepts in Modern Optics, 2010, 40(1): 163-174. [64] Yi J, Zhang B, Shao P, et al. Synthesis and photophysics of platinum(Ⅱ) 6-Phenyl-4-(9,9-dihexylfluoren-2-yl)-2,2 ′-bipyridine complexes with phenothiazinyl acetylide ligand [J]. The Journal of Physical Chemistry A, 2010, 114(26): 7055-7062. [65] Liu R, Li Y, Li Y, et al. Photophysics and nonlinear absorption of cyclometalated 4,6-diphenyl-2,2′-bipyridyl platinum(Ⅱ) com-plexes with different acetylide ligands [J]. The Journal of Physical Chemistry A, 2010, 114(48): 12639-12645. [66] Shao P, Li Y, Yi J, et al. Cyclometalated platinum(Ⅱ) 6-phenyl-4-(9,9-dihexylfluoren-2-yl)-2,2′-bipyridine complexes: Synthesis, photophysics, and nonlinear absorption [J]. Inorganic Chemistry, 2010, 49(10): 4507-4517. [67] Ji Z, Li Y, Pritchett T M, et al. One-photon photophysics and two-photon absorption of 4-[9,9-Di(2-ethylhexyl)-7-diphenylaminofluoren-2-yl]-2,2′: 6′,2″-terpyridine and their platinum chloride complexes [J]. Chemistry-A European Journal, 2011, 17(12): 2479-2491. [68] Zhang B, Li Y, Liu R, et al. Synthesis, structural characterization, photophysics, and broadband nonlinear absorption of a platinum(Ⅱ) complex with the 6-(7-benzothiazol-2′-yl-9,9-diethyl-9 H-fluoren-2-yl)-2,2′-bipyridinyl Ligand [J]. Chemistry - A European Journal, 2012, 18(25): 4593-4606. [69] Li Z, Sun W. Synthesis, photophysics, and reverse saturable absorption of platinum complexes bearing extended π-conjugated C^N^N ligands [J]. Dalton Transactions, 2013, 42(38): 14021-14029. [70] Li Z, Badaeva E, Ugrinov A, et al. Platinum chloride complexes containing 6-[9,9-Di(2-ethylhexyl)-7-R-9H-fluoren-2-yl]-2,2′-bi-pyridine ligand (R = NO2, CHO, benzothiazol-2-yl, n-Bu, carbazol-9-yl, NPh2): Tunable photophysics and reverse saturable absor-ption [J]. Inorganic Chemistry, 2013, 52(13): 7578-7592. [71] Liu X, Sun W. Platinum(Ⅱ) complexes bearing 2-(9,9-dihexadecyl-7-R-fluoren-2-yl)-1,10-phenanthroline ligands: Synthesis, photophysics and reverse saturable absorption [J]. European Journal of Inorganic Chemistry, 2013, 2013(27): 4732-4742. [72] Zhang B, Li Y, Liu R, et al. Extending the bandwidth of reverse saturable absorption in platinum complexes using two-photon-initiated excited-state absorption [J]. ACS Applied Materials & Interfaces, 2013, 5(3): 565-572. [73] Shi P, Coe B J, Sánchez S, et al. Uniting ruthenium(Ⅱ) and platinum(Ⅱ) polypyridine centers in heteropolymetallic complexes giving strong two-photon absorption [J]. Inorganic Chemistry, 2015, 54(23): 11450-11456. [74] Zhao T, Liu R, Shi H, et al. Synthesis, tunable photophysics and nonlinear absorption of terpyridyl Pt(Ⅱ) complexes bearing different acetylide ligands [J]. Dyes and Pigments, 2016, 126: 165-172. [75] Fang B, Zhu Y, Hu L, et al. Series of C^N^C cyclometalated Pt(Ⅱ) complexes: Synthesis, crystal structures, and nonlinear optical properties in the near-infrared region [J]. Inorganic Chemistry, 2018, 57(22): 14134-14143. [76] Po C, Tao C-H, Li K-F, et al. Design, synthesis, luminescence and non-linear optical properties of 1,3,5-triethynylbenzene-based alkynylplatinum(Ⅱ) terpyridine complexes [J]. Journal of Organometallic Chemistry, 2019, 881: 13-18. [77] Po C, Tao C-H, Li K-F, et al. Design, luminescence and non-linear optical properties of truxene-containing alkynylplatinum(Ⅱ) terpyridine complexes [J]. Inorganica Chimica Acta, 2019, 488: 214-218. -






 下载:
下载: